Olga Boychenko, B.A., Robert M. Bernstein, M.D., Eric S. Schweiger, M.D.
Cutis 2012; 90:73-76.
Abstract
We describe a case of a 44-year-old female with biopsy proven androgenic alopecia, nonresponsive to Minoxidil solution. After careful consideration and discussion with the patient, the decision was made to introduce oral finasteride at 1.25mg/day. After only 3.5 months of therapy there was a significant reduction in hair shedding and increased regrowth, without reported side effects. In addition, we present here a comprehensive review of the limited studies and case series that have thus far been reported using Finasteride to treat female androgenic alopecia.
Introduction
Female Androgenic Alopecia (FAGA) is the most common form of hair loss in women. Age increases the incidence with 50-75% of woman older than 65 suffering from AGA compared to 6-12% of 20-30 year olds. ((Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol 2001;144:297-304.))
The pathophysiology of FAGA is somewhat controversial. In the past, it was regarded as the female counterpart of Male Androgenic Alopecia (MAGA), and it was thought to be primarily associated with increased dihydrotestasterone (DHT). ((Kaufman, KD. Androgens and Alopecia. Molecular Cellular Endocrinology 2002; 198: 89-95.)) However, some recent studies have shown that the role of androgens in FAGA is less certain, especially in women who do not display any other signs of hyperandrogenemia. ((Futterweit W, Dunaif A, Yeh H-C, Kingsley P. The prevalance of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am Acad Dermatol 1988;19:831-6.)) The current theory is that female pattern hair loss is a multifactorial genetically determined trait with both androgen–dependent and androgen independent mechanisms contributing to the phenotype. ((Sinclair R, Chapman A, Magee J. The lack of significant changes in scalp hair follicle density with advancing age. Br J Dermatol. 2005 Apr; 152(4):646-9.)), ((Scheinfeld N. A review of hormonal therapy for female pattern (androgenic) alopecia. Dermatology Online J. 2008 Mar; 14(3):1.))
The lack of consensus among scientists on the pathophysiology of FAGA makes it a challenging condition to treat. Much emphasis has been placed on antiandrogenic agents, such as Finasteride, after seeing the success in MAGA patients. ((Rogers NE, Avrum MR. Journal of the American Academy of Dermatology 2008; 59(4): 547-66).)) Finesteride, a potent 5-alpha-reductase type II inhibitor, acts by lowering serum and scalp levels of DHT, while increasing scalp levels of testosterone. It was approved in 1997 for treatment of androgenic alopecia in men, after several double blind randomized controlled trials had proved its effectiveness.6 Finasteride is most beneficial to men with mild to moderate MAGA with 1 mg being the approved dose. ((Kaufman, KD, Olsen, EA, Whiting, D, et al: Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol 39:578–589, 1998.)), ((Leyden, J, Dunlap, F, Miller, B, et al: Finasteride in the treatment of men with
frontal male pattern hair loss. J Am Acad Dermatol 40:930–937, 2002)), ((Roberts, JL, Fiedler, V, Imperato-Mcginley, J, et al: Clinical dose ranging studies with finasteride, a type 2 5 -reductase inhibitor, in men with male pattern hair loss. J Am Acad Dermatol 41:555–563, 1999))
Since Finasteride is a category X drug, practitioners have been hesitant to use it in premenopausal women. However, within the past decade there has been increasing evidence that Finasteride does have some benefit in FAGA. Although, the role and place of antiandrogenic agents in FAGA remains to be fully defined, the use of Finasteride is increasingly gaining interest.
Case Report
A 44 year-old Hispanic woman presented to our clinic with the complaint of excessive hair shedding and thinning for over one year duration. She had no significant family history of hair loss to her knowledge. She had tried Minoxidil solution 2% for a few months without relief and had subsequently ceased using it. She denied any significant changes in weight, recent illnesses or surgeries. She also denied any new medications or recent stress. Physical examination revealed a patient who had significant diffuse thinning on the majority of her scalp her. Pull test was positive with over 7 telogen appearing hairs pulled on 4 attempts. Digital microscopic evaluation revealed around 80% miniaturization of hair follicles diffusely, with near 100% miniaturization on her frontal scalp. All lab tests were within normal limits, including androgen levels.
A diagnosis of telogen effluvium, diffuse alopecia areata and diffuse androgenic alopecia was entertained. A punch biopsy was performed and found 22 hair follicles: 13 terminal, 8 vellus and 1 telogen/catogen follicle. The terminal to vellus ratio was 1.6 to 1. There was an absence of any peri-bulbar or peri-infundibular infiltrates. There was no mucin, interface change or scarring. The dermatopathologist interpreted the findings as diagnostic for androgenic alopecia.
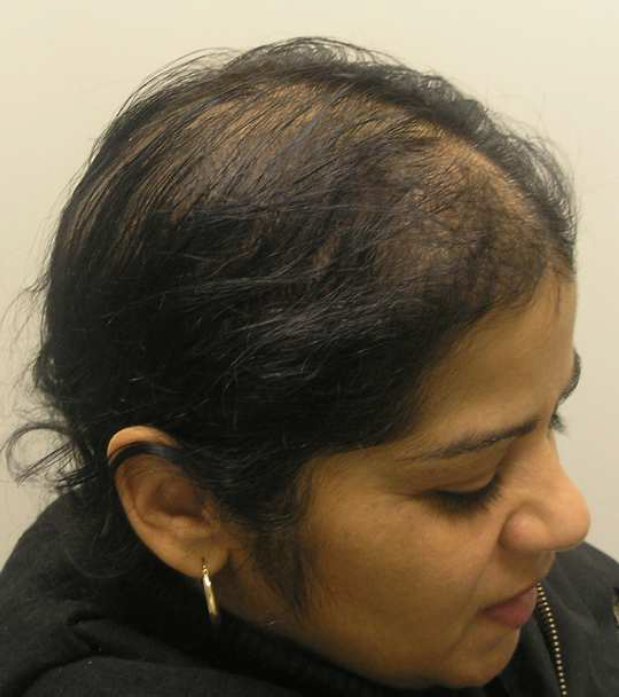
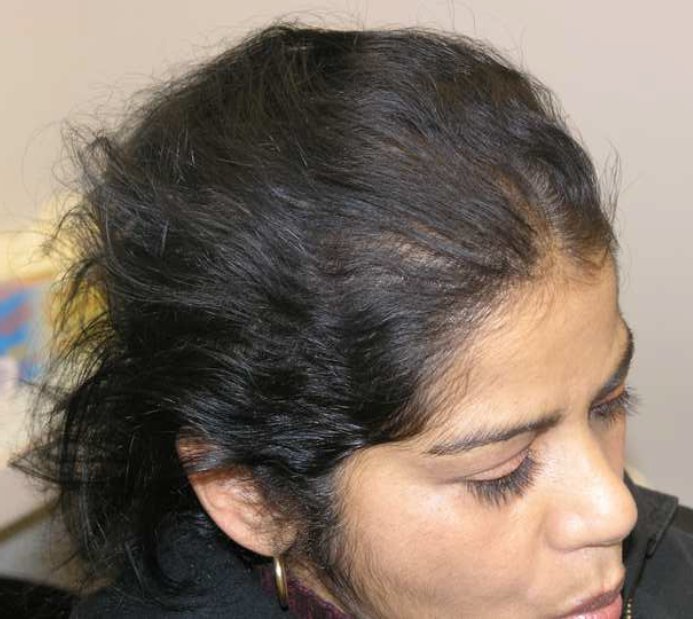
At one month follow-up the patient reported continued shedding and thinning (Figure 1a). The decision was made to initial therapy with Finasteride at a dose of 1.25 mg daily. Because of a past history of hysterectomy, no oral contraceptive was added. The patient was then seen at follow-up at 3.5 months after initiation of therapy and reported significant decreased shedding and hair re-growth (Figure 1b). She was very happy with the treatment and wanted to continue on Finasteride therapy. The decision to continue on current therapy was made.
Discussion
There is a dearth of reports in the literature reporting the use of Finasteride in the treatment of FAGA. To date, there has been only 1 randomized placebo-controlled trial, 4 case series and 1 case report published documenting the efficacy of Finasteride FAGA. ((Price VH, Roberts JL, Hordinsky M, Olsen EA, Savin R, Bergfield W, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol 2000;43:768-76.)), ((Camacho F. Hirsutismo: enfoque clinico terapéutico. Act Terap Dermatol 2001;24:190-206.)), ((Shum KW, Cullen DR, Messenger AG. Hair loss in women with hyperandrogenism:four cases responding to finasteride. J Am Acad Dermatol. 2002;47:733-739.)), ((Trueb RM. Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal women. Dermatology. 2004;209:202-207.)), ((Iorizzo M, Colombina V, Stylianos V, Piraccini BM, Tosti A. Finesteride Treatment of Female Pattern Hair loss. Arch Dermatol. 2006;142:298-302)), ((Hong JB, Chiu HC, Chan JY, Chen RJ, Lin SJ. A woman with iatrogenic alopecia responding to finesteride. Br J Dermatol 2007;156:748-791.)) The randomized controlled trial consisted of 137 post-menopausal women with normal androgen levels receiving either Finasteride 1 mg/day or placebo for 1 year.10 The results showed no improvement in slowing hair thinning, increasing growth, or improving the appearance of hair in the finasteride treated group.
The other studies shown in Table 1 did show some benefit in using finasteride. Camacho et al. treated 41 women with SAHA (seborrhea, acne, hirsutism, alopecia) using finasteride at 2.5 mg/day for 2 years, and noted improvement in hair growth.11 Shum et al. treated 4 post menopausal women with hyperandrogenism using finasteride at 1.25 mg/day for 2.5 years and observed improvements in hair loss as well as increased hair growth.12 Trueb et al. studied 5 postmenopausal women with normal androgen levels for 1 year. Four were treated with finasteride at 2.5 mg/day and 1 was treated with 5 mg/day. All 5 women improved 6 months after beginning treatment.13 Iorizzo et al. studied 37 pre-menopausal women without hyperandrogenism with finasteride at 2.5 mg/day plus an oral contraceptive (drospirenone 3 mg and ethinyl estradiol 30 micrograms) for 12 months. Improvement was observed in 23 patients, no improvement was observed in 13 patients, and 1 patient worsened despite treatment.14 Hong et al. treated a post-menopausal woman (after total hysterectomy with bilateral salpingoophorectomy) with finasteride at 2.5 mg/day.15 After the first 6 months her hair loss stabilized and by 10 months she had a noticeable increase in hair growth.
In reviewing the above studies, Finasteride does appear to be an effective treatment of FAGA in certain women, in particular those with early onset alopecia and those with increased levels of androgens. It has been used successfully in both pre- and postmenopausal women, but appropriate birth control methods must be used in the former due to the risk of feminization of male fetus. In order to have favorable results, finasteride should be given at a higher dose than that given to men, with 1.25 mg/day being the minimum.
While, sexual dysfunction (such as decreased libido, erectile dysfunction, or ejaculation disorder) has been reported in a small percentage of male patients,6 there have been virtually no adverse effects reported in females. Finasteride was well tolerated in our patient and all of the reviewed studies. It is important to note that Finasteride is not FDA approved in women and it is possible there will be unforeseen side effects reported in the future if use of Finasteride in women is increased.
In addition, Finasteride is pregnancy category X primarily because it can cause feminization of a male fetus. Therefore, appropriate birth control methods must be implemented in any women of childbearing age if Finasteride use is planned.
In conclusion, this case report and comprehensive review of the literature demonstrates that Finasteride may have a role in treating FAGA. While the teratogenic effects of Finasteride and its potential unknown long-term side effects must not be overlooked, it does appear to be effective in some women with FAGA. Further studies with varying doses and populations are needed to accurately determine the efficacy and safety of Finasteride in FAGA.
Table 1: Treatment of Female Pattern Hair Loss using Oral Finasteride
| Reference | Study Type | Women # | Androgen Level | Post- meno- pause |
Treatment Dose | Length | Primary findings |
|---|---|---|---|---|---|---|---|
| Price et al. 2000 | Double blind, randomized, controlled | 137 (E=67; C=70) | Normal | Yes | 1 mg/d | 12 mo | No difference between Finesteride group (E) and placebo group (C) |
| Camacho et al. 2001 | Case series | 41 | Increased (SAHA) | No | 2.5 mg/d | 2yrs | Improvement in all 41 women with seborrhea, acne, hirsutism, alopecia (SAHA) |
| Shum et al. 2002 | Case series | 4 | Increased | Yes | 1.25 mg/d | 2.5yrs | Improvements in all women in both hairloss and hair growth |
| Trueb et al. 2004 | Case series | 5 | Normal | Yes | 2.5 or 5 mg/d | 12 mo | All women improved 6 months after initiation of treatment |
| Iorizzo et al. 2006 | Case series | 37 | Normal | No | 2.5 mg/d + drosperinone + estradiol |
12 mo | Improvement in 23 women, no improvement in 13 women, 1 patient worsened despite trx |
| Hong et al. 2007 | Case report | 1 | Increased | Yes | 2.5 mg/d | 10 mo | Hair loss stabilization after 6 months, hair regrowth after 10 months |
| Boychenko et al. 2009 | Case Report | 1 | Normal | No | 1.25 mg/d | 3.5 mo | Hair loss stabilization and significant regrowth |